The World of Oil and the Petrochemical Industry #2
Hello guys. While scrolling through my past posts on steemit, I stumbled across one that really caught my attention. It's a post on The World of Oil and the Petrochemical Industry written almost 8 months ago. At the end of that post, I left a promise that I would continue the post later on, but which I later forgot to. So, here I am today, writing the sequel to the post in fulfilling my promise of yestermonths with joy.
So, let's start...........
THE HYDROCARBONS IN CRUDE OIL
Crude oil is a mixture, and because of this its composition varies. Crude oil is composed mainly of hydrocarbons, and we have already seen that there can be several hundreds of these because of carbon’s ability to form stable chains, branched chains and rings. No two deposits of oil contain exactly the same hydrocarbons in the same proportions. However, we can divide the hydrocarbons found in oil into three major classes of compound: alkanes, cycloalkanes and arenes (also known as aromatic hydrocarbons).

Image by Nicola Giordano from Pixabay
ALKANES
Alkanes are saturated hydrocarbons. They can either be branched chains or straight chains. Examples of which are methane, ethane, propane, butane, methylpropane, etc. Merely looking at the molecular formulae of the alkanes, and you could spot a mathematical relationship. For every carbon atom in each formula, there are twice that number of hydrogen atoms, plus another two. This can be represented by the general formula CnH2n+2, in which n is the number of carbon atoms. Let’s try out this general formula with methane:
n = 1 because there is one carbon atom. So the molecular formula for methane is:
C1H(2 × 1)+2 = CH4
CYCLOALKANES
Most of the cycloalkanes found in crude oil are based on five- or six-membered carbon rings, as shown in the figure below:
Cyclopentane
Cyclohexane
ARENES
Arenes or aromatic hydrocarbons contain at least one benzene ring. The name ‘aromatic’ originally came from the characteristic smell of some naturally occurring compounds that contain a benzene ring. I wrote a post in the past to extensively discuss about arenes. (You can check here and here).
Peak oil
It may seem obvious, but at some stage the world production of oil has to reach a peak. Dr M. King Hubbert, a US scientist, first put this idea forward in 1956. The graph he produced is called Hubbert’s Curve. He used his curve to accurately predict the decline in US oil production in the 1970s. Now, many scientists believe we may reach a peak in oil production by 2010, after which oil production will start to decline. In 2007 the world produced almost 90 million barrels of oil a day. Each barrel contains 159 litres. This means that every second our world consumed 166 000 litres of oil.
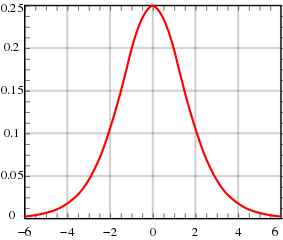
Our thirst for oil is increasing as the economies of China and India develop at a rapid rate and older industrialised nations, such as the UK and USA, fail to reduce their demand. But not all scientists believe we will reach Hubbert’s peak. As the price of oil spirals ever upwards there will be new incentives to find new oil fields and produce more oil from existing ones. However, if oil production does peak as predicted, then the world faces a much more uncertain future, albeit there are other alternatives to oil around now.
MAKING CRUDE OIL USEFUL
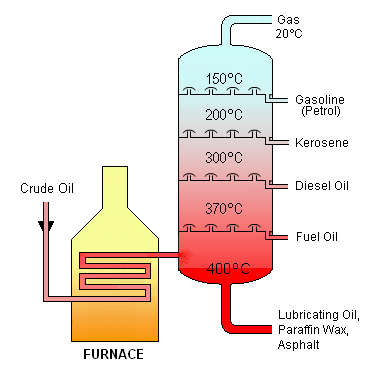
Crude oil as it comes out of the ground is of little practical use. To make it useful, it must first be separated into its components, which is done by fractional distillation.
FRACTIONAL DISTILLATION
Fractional distillation is the separation of a mixture of compounds by the different boiling points. When crude oil is separated there are five major fractions, as shown in the table below.
The major fractions of crude oil and their uses
| Fraction | Boiling point range/°C | Number of carbon atoms | Percentage of crude oil | Uses |
| refinery gas | less than 20 | C1 to C4 | 1 to 2 | Fuel and as a feedstock for petrochemicals. |
| gasoline/naphta | 20-100 | C5 to C12 | 15 to 30 | Petrol for transport and as a feedstock for petrochemicals; (the part of the fraction so used is called naphtha). |
| kerosene | 120-220 | C10 to C16 | 10 to 15 | fuel for jets, paraffin for heating. |
| diesel oil | 220-350 | C15 to C25 | 15-25 | Fuel for transport, power plants and heating. |
| residue | more than 350 | > C2 | 40 to 50 | Oil-fired power stations, polishing waxes, lubricating oils, bitumen on roads. |
When crude oil reaches a refinery it is heated to about 400 °C and much of it vaporises. As vapour and liquid, it is fed into a fractionating column, which is about 60 metres high. The liquid collects at the bottom of the column and is called the residue. The vapour passes on up the column and as it cools it turns back to liquid, collecting in trays at various heights. The smaller the vapour molecules, the further the vapour can travel up the column before it condenses. This is because smaller molecules form liquids with lower boiling points. At the top of the column, gases that contain very small molecules are collected. These hydrocarbons boil below 20°C.
This is the first distillation – the primary distillation – and the fractions coming from the column are further separated by a variety of processes. Before a fraction can be used, the sulfur must be removed (this forms a valuable by-product).
MEETING THE DEMAND: USING ALL THE FRACTIONS
The gasoline fraction is the main source of petrol for car and other vehicle engines, but the amount of gasoline fraction produced is never enough to meet the demand for petrol. Typically, about 40 per cent of the output from a distillation column may be required as petrol. Compare this percentage with that given in the table above for the gasoline fraction. So, other fractions have to be altered by chemical processes to produce the extra petrol needed.
CRACKING LARGER MOLECULES
Petrol for use in cars and other vehicles requires alkanes in the range from C5 to C10: To meet demand, longer alkanes from other fractions are shortened by being split. This process is known as cracking. There are two basic processes for splitting alkane chains:
- using high temperatures and pressures, which is called thermal cracking:
- using catalysts, which is called catalytic cracking (or ‘cat cracking’ for short), where the pressure is slight but the temperature is still high.
Whichever process is employed, sufficient energy must be supplied to split the very strong C-C and C-H bonds. The advantage of thermal cracking is that molecules in the residue can be cracked. Catalytic cracking only works on the distillate (the liquid that has been distilled) such as that from the diesel oil fraction. However, catalytic cracking tends to produce more branched-chain alkanes, which are better for use in petrol as they promote efficient combustion. Take, for example, the molecule of dodecane, C12H26: notice that in this case the molecule has been split and a branched-chain alkane produced. Branched chains are useful in petrol blending because they have higher octane numbers. Of course, the molecule can split in almost any way, so a huge variety of hydrocarbons, both straight chains and branched, are produced. These are separated by fractional distillation.
Bond breaking in thermal and catalytic cracking
The main difference between thermal cracking and catalytic cracking is the way in which covalent bonds in the carbon chains are broken. A covalent bond consists of a pair of electrons that is shared between two atoms. The splitting or fission of the covalent bond in thermal cracking leaves each species produced with one electron of this pair. This type of bond splitting is called homolytic fission.
When a species has an unpaired electron it is called a free radical (or sometimes just radical).The unpaired electron makes the free radical very reactive and in the reaction mixture it will react again to produce either an alkane or an alkene.
Thermal cracking requires high temperatures (between 400 and 900 °C and high pressures (up to 7000 kPa), so it is expensive. On completion, it produces a high percentage of alkenes, which are used to produced most of the organic chemicals on which our modern world relies.
Catalytic cracking involves a different type of bond splitting known heterolytic fission. In this case the shared pair of electrons both go to one of the species produced when the covalent bond is broken. A positive and a negative ion are produced. Heterolytic fission produces a positive and negative ion. The positive ion is called a carbocation. This reacts with other alkane molecules to produce a high percentage of branched-chain alkanes, which are ideal for use in petrol. Catalytic cracking is carried out at about 450 °C with a slight pressure and is the major method of producing petrol and also aromatic hydrocarbons.
REFORMING AND ISOMERISATION
Catalytic reforming is used to modify molecules to suit demand. Straight- chain alkanes can be converted into cycloalkanes and arenes. Different catalysts are used, depending on the product required. The catalysts used are often bimetallic catalysts such as platinum-rhodium. An example is given in the catalytic reforming to produce cycloalkanes and aromatic compounds. It is observed that during reforming, whatever happens to the molecule, the total number of carbon atoms remains the same. I have already mentioned the need to produce branched-chain alkanes to increase the octane number of petrol. This can be done by taking a straight chain alkane, heating it to break one of the C-C bonds and allowing the molecule to reform, which often produces a branched chain. This process called isomerisation.
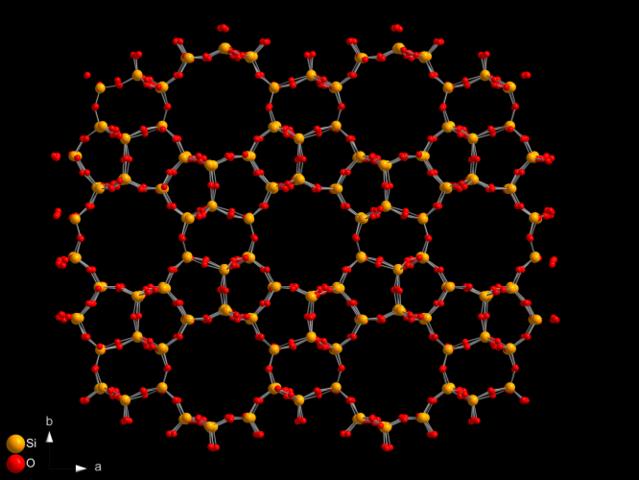
Zeolites
Zeolite means ‘boiling stone’ (from the Greek zeein for boiling and lithos for stone). The name was coined by a Swedish scientist, Baron Cronstedt, in 1756, when he discovered a mineral that released steam and appeared to boil when heated. This name now applies to about 40 naturally occurring minerals that display the same property when heated. Many more zeolites are made synthetically. The steam comes from water trapped in tiny pores and channels in the zeolite. These channels are the key to understanding how this remarkable substance is such an effective catalyst. It took some 200 years from Cronstedt’s discovery until synthetic zeolites were first used commercially in 1959 to crack hydrocarbons.

Natural zeolite. United States Geological Survey, Public Domain
Zeolites are a family of aluminium silicates. Their silicon, oxygen and aluminium atoms provide a regular network of pores and interconnecting channels, rather like a sponge. The size of the pores is critical to the use of zeolites as catalysts for the host chemicals. Pore sizes (in picometres, 10-12 m) range from 300 pm to 1000 pm, which is why the pores trap water molecules (which have an effective diameter of about 270 pm). If the pore size is large enough, the zeolite can accommodate hydrocarbon molecules, which are cracked inside the structure.
Synthetic rather than natural zeolites are now used for catalytic cracking, and there is much research into ways to alter the sizes of pores and channels by inserting different atoms into the structure. Zeolites have saved the oil industry billions of pounds because they are so efficient and produce more commercially desirable products than the clay catalysts previously used.
The uses for zeolites don’t stop with cracking. In New Zealand, which has vast reserves of natural gas but hardly any oil, a synthetic zeolite, ZSM-5, converts methanol into petrol. Methane is first converted into methanol, then ZSM-5 removes water from the molecules to leave hydrocarbon chains trapped inside the zeolite. These are then driven out of the zeolite as petrol.
Zeolites in washing powder
Almost certainly you have zeolites in your house. Look at a packet of washing powder and you will probably find more than a quarter of the powder is made from zeolites. The function of zeolites here is to remove calcium ions, which cause water hardness, and replace them with sodium ions, which soften the water. About three-quarters of the world’s production of zeolites ends up in detergents.
Zeolites also function as molecular sieves. For example, detergents need to be biodegradable. The largest part of the detergent molecule is a hydrocarbon chain. If this chain is branched, microorganisms at the sewage works cannot break down the detergent. At the refinery, unbranched hydrocarbons for detergent manufacture are separated from those with branched chains by passing them into the channels of a zeolite. The branched chains are too large to enter the zeolite, so just the unbranched hydrocarbons pass through it.
There is speculation that zeolites may have provided the organising influence that produced the biologically active molecules that form the basis of life on Earth.
PETROCHEMICALS
More than 90 per cent of crude oil is burnt; the rest forms the basis of petrochemical industry, which produces almost all of our organic chemicals. I have already said that alkanes are rather unreactive.

However, the cracking process produces small-chain alkenes, of which
ethene is the most important. Ethene provides a synthetic route to many organic
chemicals. The reactivity of its double bond provides a way to insert many different
functional groups and thereby allow the full potential of hydrocarbons to be
realised.
SUMMARY
After studying this article and the previous one (check here), you should know that:
- Carbon is unique in its formation of stable chains produces and rings, millions of different chemicals.
- Crude oil is a hydrocarbon mixture of alkanes, cycloalkanes and arenes (aromatic hydrocarbons).
- Components of crude oil can be separated by fractional distillation in a fractionating column. The five major fractions separated in a fractionating column are refinery gases, gasoline (includes petrol and naphtha), kerosene, diesel oil and residue.
- Larger hydrocarbon molecules are broken down (cracked) into smaller ones for petrol and to produce alkenes, such as ethene, for the petrochemical industry.
- Thermal cracking produces a high percentage of alkenes via a free radical mechanism. It takes place at high temperature and pressure.
- Catalytic cracking produces branched-chain alkanes suitable for motor fuel and aromatic hydrocarbons. It takes place via a carbocation mechanism. It still uses a high temperature, but only slight pressure.
- Reforming is a way to produce cycloalkanes and arenes from straight-chain alkanes.
- Crude oil is a finite resource. Its use as a feedstock for most organic chemicals has changed the world, yet most of it is burnt as fuel.
- Saturated hydrocarbons have no double or triple carbon-carbon bonds in their molecules.
- Unsaturated hydrocarbons possess double or triple carbon-carbon bonds.
Thanks for reading.
REFERENCES
https://www.bbc.co.uk/bitesize/guides/zxd4y4j/revision/1
https://www.britannica.com/science/crude-oil
https://en.wikipedia.org/wiki/Alkane
https://en.wikipedia.org/wiki/Aromatic_hydrocarbon
https://www.chemguide.co.uk/organicprops/arenes/background.html
http://klemow.wilkes.edu/Hubbert.Curve.html
https://en.wikipedia.org/wiki/Hubbert_peak_theory
https://en.wikipedia.org/wiki/Hubbert_curve
https://en.wikipedia.org/wiki/Peak_oil
https://www.newworldencyclopedia.org/entry/Fractional_distillation
https://study.com/academy/lesson/what-is-fractional-distillation-definition-process.html
https://en.wikipedia.org/wiki/Fractional_distillation
https://www.bbc.co.uk/bitesize/guides/zshvw6f/revision/5
https://www.chemguide.co.uk/organicprops/alkanes/cracking.html
https://en.wikipedia.org/wiki/Cracking_(chemistry)
This post has been rewarded with an upvote from city trail as part of Neoxian City Curation program
 . We are glad to see you using #neoxian tag in your posts. If you still not in our discord, you can join our Discord Server for more goodies and giveaways.
. We are glad to see you using #neoxian tag in your posts. If you still not in our discord, you can join our Discord Server for more goodies and giveaways.
Do you know that you can earn NEOXAG tokens as passive income by delegating to @neoxiancityvb. Here are some handy links for delegations: 100SP, 250SP, 500SP, 1000SP. Read more about the bot in this post. Note: The liquid neoxag reward of this comment will be burned and stake will be used for curation.
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.