Teaching Science practical lesson 2 with my Students || How Simple Distillation is performed
(Edited)
Greetings to Y'all!
Very soon the Basic Examination Certificate Examination would be written by our Candidates in JHS 3. The month for writing every year has been shifted from November to August 2023, which is very closed. As science teachers we have a lot to cover with our candidates. One of the key areas we are supposed to touch is the practical aspect which is compulsory. The students are to answer four (4) compulsory practical questions in order to test their knowledge on field work, especially being conversant with some of the things they would do at the field.
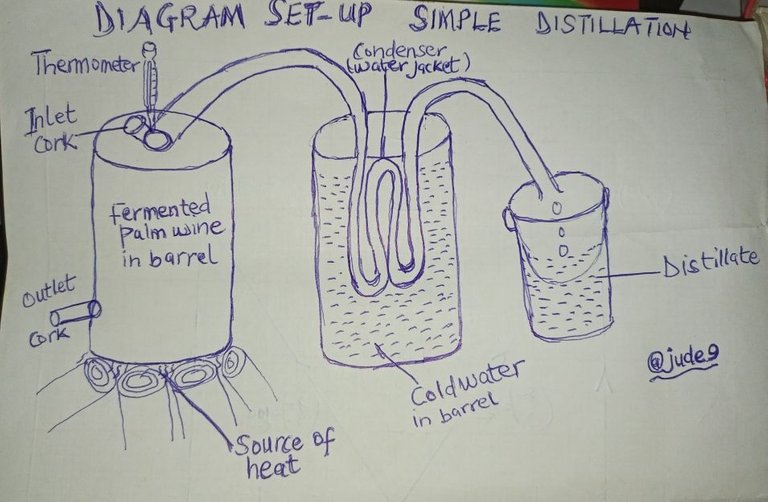
I decided to tackle on "Simple Distillation" which is one area that students find it very difficult. It's separation of two mixtures with miscible mixtures with different boiling points. For instance, Ethanol (Alcohol) and water. Alcohol boils at 78°c while water boils at 100°c. So, it's easy to separate them. This isn't something cumbersome since we can make improvisation of local materials around.
"To start with......"
When we well young, at our local village, our fathers used to send us to the palm wine tappers to collect palm wine for them to drink. I mean the fresh one which is just collected as soon as the palm trees is prepared for drawing of palm wine. It was later that I learnt that the palm wine we used to drink is a mixture of alcohol and water that is why the alcohol is being able to extract from the water content.
As I used to visit the territory of palm wine tapping, the palm wine tapper would collect the palm wine in a large enclosed barrel for about a month, since the palm draws from the palm trees into that local pot in drops. So, the palm wine tapper has to exercise with patience. When the barrel is full or when it gets to the level he wants to be used, the process of distilling the alcohol from the water begins. By then the palm wine has been fermented.
Only three set ups is needed for the completion of this local way of how alcohol is extracted from the palm wine. Fire is set at the bottom of the barrel containing the fermented palm wine. The palm wine tapper makes sure that the cork or lid at the top ( inlet ) is well closed in order to prevent any vapour from coming out. The second point of hole beneath the base of the barrel ( water outlet) is also closed with some improvised wooden cork. This is where the water from the palm wine would be drained out after the alcohol has been exhausted from the barrel leaving the water. Thermometer is supposed to be placed at the enclosed top lid or cork to monitor or check the temperature at which the water from the palm wine would begin to boil so that the distillate would be ceased to collect. But, the local palm wine tappers use their experience and imagination. However for scientific practical purposes we need to use thermometer.
There's a metal pipe( tube) which is mostly coated with zinc to prevent frequent rusting as liquid and oxygen are the main agents for rusting. this metal pipe is extend to the second barrel that contain cold water ( water jacket). The metal pipe in this cold water is coiled (wounded) severally so that it has the tendency to cool the vapour that passes through the metal pipe. Like I have mentioned, the main function of this station barrel with cold water would serve as water jacket to cool all the vapour of alcohol passing through the metal pipe. This second point of barrel sometimes become very hot to the extent that the water in it boils. So, the palm wine tappers normally add up fresh cool water as most of it evaporate and the level of the water decreases.
The final station or point is where the distillate is collected as it cools it turns into liquid. So, the metal pipe from the water jacket extends to to the last container where the alcohol (distillate is collected. So, when the fermented palm wine boils to 78°c the water content in the fermented palm wine would begin to boil at 100°c. So, there isn't need to wait till 100°c before removing the lid or cork from the base of the boiling palm wine contained in barrel. The lid is then removed to let all the water to drain out from the barrel. Alcohol collected in this form contain 100°c alcoholic content so they have to dilute is with water to reduce the concentration before people can be taken.
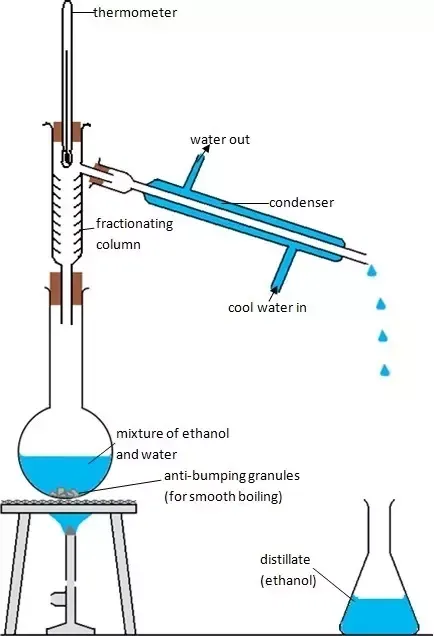
This improvisation of how simple distillation is performed is the same as what is observed in commercial base production of ethanol. It's very imperative for students to get the whole picture at their local level than to complicate the whole content which makes it very difficult for understanding. I believe with this "science practical made simple, my students would be able to grasp and deliver when they meet such questions in their exams.
333
1
288.032 NEOXAG
I wanna try lavender oil destilation sometimes in future, looking for the diy tips and tricks.
Literally your post came at the right time. 😂
Thanks for loving my post. I hope it will be useful here in hive blog.
Certainly it will be to me!
👌🏻
https://twitter.com/1145046831635816448/status/1637338490097598465
The rewards earned on this comment will go directly to the people sharing the post on Twitter as long as they are registered with @poshtoken. Sign up at https://hiveposh.com.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.