Explaining Chemistry In Simple Words (Part 1)
Chemistry is fun, although can be very boring if you attended the type of high school I did where there are no practicals with everything being theory. I got to the university before I began to enjoy chemistry and I realized that practical would have done me more good if I had started early enough.
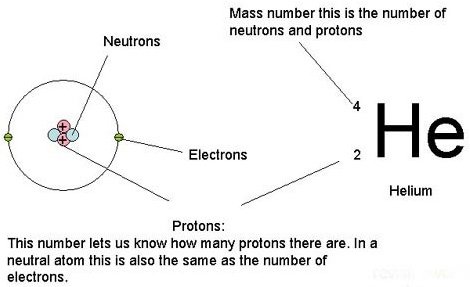
That said, chemistry is quite simple and easy and it can be explained to everyone around in simple terms, just the same way physics and biology has to do with everything in our world, so does chemistry. Everything in our world are made up of Atoms which is made up of a nucleus and electrons with the nucleus being the core. The Nucleus is made up of proton and neutron and different elements are determined by the number of protons that are present in them.
wikimedia
Just like you learned in high school, Atoms have multiple electron shells, and the electrons at the outermost shells are regarded as Valence electrons and you see, the majority of what happens in chemistry and with the elements are determined by these valence electrons and if you look at the periodic table, all electron found in the same group or column have the same valence electron (equal number of electron in their outer shell). All elements in the main group except for helium fall between 1 to 8, and Helium is an exception because it is too small with just 2 electrons, yet still acts similarly to noble gases and there are still other element in groups like the transition metals.
Just like I was thought in high school, all elements of the same valence electron react equally, and behave the same. If you remember clearly, elements in the first group are known as Alkaline Metals off-course without hydrogen, and these elements are soft shiny metals with 1 valence electron, and they can be explosive, and they react with water and air.
Okay, I have explained groups. All element in the same period have the same number of shells which increases from top to bottom and when it comes to the mass of the elements, they increase according to how the elements move from left to right because the elements gain proton, neutron, and electron. Talking about neutron, an isotope is determined by the number of neutrons in the nucleus and this is why we can have the same elements having different isotope.
That said, you might have heard that an atom is either positively charged, negatively charged, or just neutral. Well, allow me to explain in simple terms. If an atom has equal number of protons and neutrons, then it has no charge and so neutral. When it has more proton than neutron, it is positively charged and when it has more neutrons than protons, then it is negatively charged. Don't you think I deserve an Energy drink for the explanation so far? Well I bought a Monster Energy before I started the post, and I am yet to finish it so thanks but you can give me an upvote you know, it will encourage me.
Back to chemistry. Atoms that are charged are known as Ions and when it is negatively charged, it is referred to as Anion and when it is positively charged, it is referred to as Cation, the difference is the prefix. On the periodic table, we could see elements in three groups which are metals, non-metals (gases) and those in-between known as semimetals. So now that we know that we have atoms, what do we call two or more same atoms that bond together?
They are referred to as a molecule (H + H = H2) but this isn't the same when there are different atoms because what we get is a compound (H2 + O = H2O )and not a molecule but so you know, compounds do not act like the compounds they are made off for instance, Hydrogen is flammable and oxygen make other materials burn easily but the combination of both elements to form a compound that completely doesn't help combustion. Another example is Sodium which is an explosive metal combined with Chlorine which is a toxic gas to give Sodium Chloride which is a Table salt.
I want to explain Isomers, Molecular Formula, Lewis Dot Structure, Atomic Bonds and A whole lot more but I am out of my energy drink so I will do it in the next post. Actually I am not out but I want to be able to explain what you need to understand when the subject chemistry is mentioned to you properly so I decide to end the post here today to continue in my next post. So expect more while I sign out from my chemistry lab where I have been mixing this post for you.
Reference
https://www.arpansa.gov.au/understanding-radiation/what-is-radiation/ionising-radiation/atomic-structure
https://uen.pressbooks.pub/introductorychemistry/chapter/the-structure-of-the-atom
https://chemistrytalk.org/what-are-valence-electrons/
https://chem.libretexts.org/Courses/Furman_University
https://www.acs.org/education/whatischemistry/periodictable.html
https://www.amnh.org/explore/ology/physics/how-to-read-the-periodic-table
https://byjus.com/chemistry/elements-and-compounds/
https://study.com/learn/lesson/what-is-an-ion-examples.html
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.