Chemistry of the cosmos (Part-3)
Much study, beginning in 1885, was devoted to the study of the distribution of elements in the universe. We can now present some general considerations due to the efforts of many researchers. The degree of dispersion of a given element is determined by the stability of the atomic nucleus on the one hand, and the speed of the nuclear reactions that give rise to the nucleus in question on the other (Lawrence H. Aller).
The most common elements, oxygen, silicon, magnesium, sulphur, calcium, nickel, and iron, all have even atomic numbers. Isotopes with an even number of neutrons are found more frequently than isotopes with an odd number of neutrons. It is now widely accepted that hydrogen and helium are the most abundant elements in the universe (after dark matter). Carbon, nitrogen, oxygen, and neon follow in that order.
- Lawrence H. Aller discovered that the degree of element dissemination grows exponentially as the atomic mass increases and reaches a value close to 100; beyond this, the degree of dissemination decreases. There is a proliferation of the corresponding elements in the atomic mass range of 53 to 63. (the iron peak). Isotopes with atomic masses divisible by four have more common nuclei than other isotopes. In general, elements with atomic masses that are divisible by two are more common. The nuclei of heavy element isotopes are typically richer in neutrons, while proton-rich nuclei are uncommon. The nuclei of the various elements are thought to be formed in nuclear reactions occurring within stars.
Any star originally consisted of hydrogen, which was converted into helium at a rate that produced energy equal to 26.7 MeV. (2 percent come back to the losses by formation of Neutrinos).
The conditions for the synthesis of various constituent nuclei in the interior of a star can alter dramatically as the star evolves. When virtually all of the hydrogen in a star's core has been converted to helium, the star begins to compress that hydrogen, raising core temperature, speeding up the carbon-nitrogen cycle, and increasing the relative quantity of the 13C isotope. Beginning with the emission of the appropriate rays, beryllium is formed when helium nuclei combine. Three beryllium nuclei fuse to form carbon 12C, and two beryllium 8Be nuclei interacting with helium nuclei also result in the creation of the same type of nucleus. Following transformations are expected to produce magnesium, sodium, and other elements:
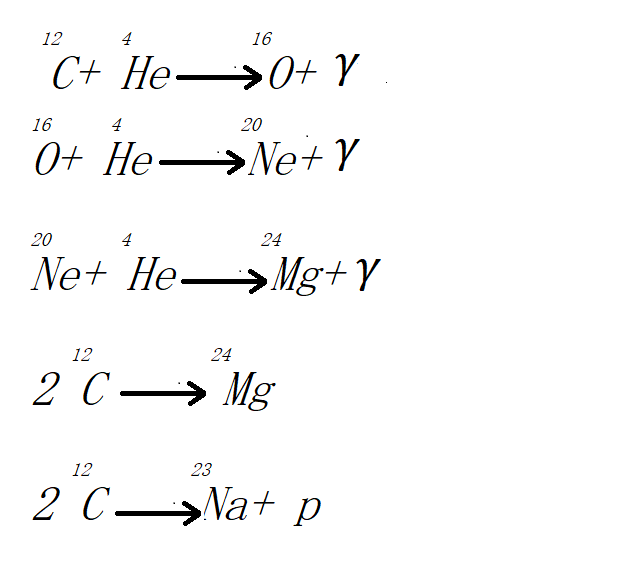
[Created by using microsoft paint]
Under certain conditions, carbon, neon, and other elemental nuclei can capture protons and transform into heavier nuclei. Massive stars lose some of their mass by ejecting heavy element atoms. There is no doubt that helium nuclei can participate in reactions that produce silicon, sulfur, argon, calcium, and other nuclei.
What then is the direction of the evolution of cosmic processes?

Source of picture: Pixapay
Can we predict the direction of this evolution using our most general laws, the principles of thermodynamics? Unfortunately, we can only provide a negative response. The principle of increasing entropy is difficult to apply to a system like the universe. Indices indicating a tendency toward equilibrium are impossible to detect in this system. The birth of new stars and the extinction of old stars, the increase in potentials, and the persistence of various dissipation processes do not allow the cosmos to be considered an equilibrium system. To get out of this situation, one must consider gravitational fields, which cannot be included in a closed system. The increase in entropy in the latter case is no longer linked to the establishment of a statistical equilibrium.
Another significant difficulty is that all laws of mechanics are reversible with respect to time; that is, by replacing the plus sign with the minus sign in front of the time variable, we do not change the laws of mechanics in any way. If we accept that the most likely variation of a given microscopic state corresponds to a transition to a higher entropy state, a change in the sign of the variable time will allow us to obtain the state of the system that preceded our current system, which will also have a high entropy value. This, however, contradicts the experimental findings.
The most subtle aspects of atom structure, such as the interactions of the nucleus and electrons, make an unexpected contribution to the solution of the universe's complex problems. As an example, consider the possibilities provided by cosmic soundings via radiation with a wavelength of 21.1 cm. The spin of a hydrogen atom's nucleus can be parallel or antiparallel to the spin of the electron. Because the magnetic moments of the particles are oriented in opposite directions in the first case, the force of attraction between the nucleus and the electron is greater than in the second case (the particles carry charges of opposite signs ). As a result, an atom with parallel spins and an atom with antiparallel spins occupy different energy levels, and transitions between them must be accompanied by energy emission or absorption. This transition's energy quantum corresponds to a wavelength of 21.1 cm. This transition is a rather rare phenomenon, occurring on average for a hydrogen atom only once every 50 million years or so, but because the number of atoms is so large, the emissions on this wavelength can easily be observed. Measurements at this wavelength enabled scientists to determine the density of hydrogen in the universe, the temperature in the universe's spaces, the speeds of stellar clusters, and so on.
References:
Interstellar Atoms and Molecules
Star Formation- University of Rochester
Interstellar medium-Wikipedia
THE COSMIC CHEMICAL BOND-David Arnold Williams T W Hartquist
EARTH AND COSMOS-Robert S Kandel
Radiation as a Constraint for Life in the Universe-Ximena C.AbrevayaBrian C.Thomas
What elements make up the Sun?- Kristine Spekkens
Our Sun- NASA science
- Apfel, Necia, Nebulae: The Birth and Death of Stars, 1988, Lothrop, Lee and Shepard, ISBN 0-688-07229-1. Explains the life cycle of stars to upper elementary school students and above (Book).
The Chemical Composition of Stars and the Universe
Comets- NASA science
White dwarf
Lots of hydrogen around! Thanks for the text
!1UP
You have received a 1UP from @gwajnberg!
@stem-curator, @vyb-curator, @pob-curator, @neoxag-curator
And they will bring !PIZZA 🍕.
Learn more about our delegation service to earn daily rewards. Join the Cartel on Discord.
PIZZA Holders sent $PIZZA tips in this post's comments:
@curation-cartel(8/20) tipped @benainouna (x1)
Learn more at https://hive.pizza.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.
Be careful, this is the amount of energy released by one fusion reaction. This is not the energy that is released by the star when converting all of its hydrogen.
And now, let me move on with a funnier comment:
This wavelength corresponds to the only transition in the spectrum of the most abundant element of the universe which is observable under the prevailing low temperature conditions in the universe. This wavelength is thus considered as a remarkable characteristic of the universe, and is as such used in extraterrestrial intelligence research. Aliens with access to technology should know it. For that reason, it was then used in the messages shipped with the Pioneer and Voyager spacecrafts.
I read something like this in the sources I looked at, but wasn't sure of it. So I will take the opportunity to ask you because you are an expert in this field. After research and messages shipped with spacecraft, has it really been found that aliens exist?
Thank you, sir, for your valuable comments
The answer is no. There is no sign that the messages in the spacecrafts have been delivered to anyone.
Cheers!
Thank you Sir.
Have a nice day
Dear @benainouna, we need your help!
The Hivebuzz proposal already got important support from the community. However, it lost its funding a few days ago and only needs support to get funded again.
May we ask you to support it so our team can continue its work?
You can do it on Peakd, Ecency, Hive.blog or using HiveSigner.
https://peakd.com/me/proposals/199
All votes are helpful and yours will be much appreciated.
Thank you!