Chemistry of the cosmos (Part-2)
The most widespread element in the cosmos:
Hydrogen is the most abundant element in the universe. It is the primary component of our Sun and many stars.
Stars are composed of 35 to 90 % hydrogen by volume. In interstellar space, hydrogen accounts for nearly 90% of the dispersed matter, with helium accounting for 9% and oxygen, nitrogen, carbon, and neon accounting for about 1%.
solar flare- Source of picture: Pixapay
70% hydrogen, 28% helium, 1.5 percent oxygen, nitrogen, and carbon, and 0.5 percent other components form the sun. Hydrogen, helium, strontium, calcium, and iron lines are plainly visible in the sun's spectrum.
The atmospheres of the large planets contain, in addition to hydrogen, significant quantities of methane and ammonia.
Analysis of emission spectra from stars, gaseous atmospheres and comet tails demonstrates the presence of diatomic molecules C2 of carbon, carbon monoxide, heavy metal oxides (titanium, zirconium, etc.).

Source of picture: Pixapay
Comets are an important source of matter dispersed in space. The solid masses, the (ices) of comets, melt, evaporate, and disperse as they approach the sun. These processes are accompanied by real explosions, which contribute to matter dispersal.
Comets frequently contain massive amounts of hydrocarbons, so they can be significant suppliers of organic compounds, competing with all terrestrial sources of hydrocarbons in this regard.
White dwarfs:
- The matter of cosmic bodies can be found in very different states than those found on Earth, and the universality of Mendeleev's periodic system does not preclude the study of star composition from revealing chemical surprises.
One of these unexpected discoveries was the discovery of (white dwarfs). This is referred to as stars, and they have dimensions hundreds of times smaller than the Sun, but their density is extremely high, on the order of hundreds of kilograms per cubic centimeter.
Sirius is one of the known white dwarfs. A glass of water, for example, would weigh 20 to 30 tons on a white dwarf. White dwarfs are gaseous stars, despite their enormous density. The atoms of this degenerate gas lack electrons; after the dispersion of the electronic envelopes, an enormous pressure compresses the atomic nuclei. The diameter of an atom, including its electronic shell, is of the order of 10 to the power minus 8 cm. The core diameter is only of the order of 10 power minus 12 cm. As the volume is proportional to the cube of the radius, we see the enormous densification which can result from a compression of atoms totally or partially devoid of electronic shells.
The universe is highly dynamic. The enormous masses of stars brought to temperatures of several million degrees, which constantly eject flow of atoms, protons, and electrons, move through space filled with rarefied and cold gas, dust particles, and fragments of asteroids, comets.
What, then, is the origin of these contrasts, the source of energy that has kept stellar matter in this state of high activity for billions of years?
Calculations show that the effective energy release is several million times greater than that obtained if the energy source is a combustion reaction.
Physics discovered that matter transformations are accompanied by an increase in energy on the order of the processes that occur within stars. These are nuclear reactions. The nuclei's transformations are frequently linked to mass variations, such that the mass of the nuclei produced is less than the sum of the masses of the initial nuclei. The variation of a system's mass m results in a variation of its energy equal to mc to the power of 2, c being the speed of light, according to Einstein's law. As a result, if the mass of the reacting nuclei decreases by 1g, the system releases 9.10 to the power of 20 ergs of energy.

Source of picture: Pixapay
As a result, bodies that participate in nuclear reactions that release large amounts of energy serve as stellar fuel, capable of maintaining the thermal regime at high temperatures despite significant energy dissipation due to radiation in the cold space around them.
Nuclear reactions, unlike elementary chemical reactions, result in elemental transformations. As a result, the composition of stellar matter is constantly changing.
The reaction of interaction of two protons, which occurs at a temperature of approximately 15 million degrees, is the simplest process of nuclei transformation. The protons cannot approach each other under normal circumstances because powerful repulsive forces exist between their charges of the same sign. The energy of thermal agitation becomes sufficient to overcome the barrier created by repulsive forces at the temperature indicated above, and the protons react according to the equation:
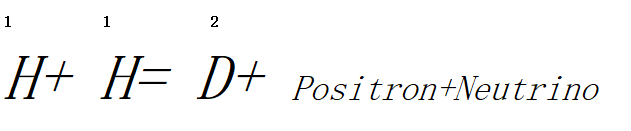
[Created by using microsoft paint]
A proton is converted into a neutron and a positron in this process and a deuterium nucleus is obtained. This new nucleus then reacts with a proton, producing a three-mass helium isotope.
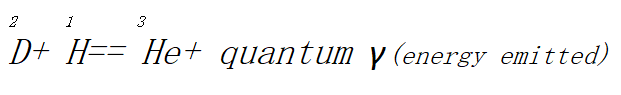
[Created by using microsoft paint]
Finally, the final reaction occurs between two helium 3 nuclei to produce the usual helium 4He and two protons:
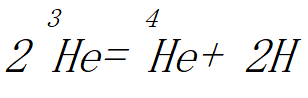
[Created by using microsoft paint]
The Sun's energy source is this series of reactions; the reason for this is that the total mass of four protons is slightly less than the mass of the helium nucleus, and it is precisely this mass defect that determines the production of enormous amounts of solar energy.
Another series of reactions is taking place in the depths of the stars, where the temperature has already reached hundreds of millions of degrees. While the beginning and last phases of this new series of reactions are identical to those of the previous series, the intermediate stages ensure the creation of nuclei of nitrogen, carbon, and oxygen isotopes, giving the process a cyclical nature.
This type of reaction is a catalytic process in which the intermediate product is obtained and consumed at the same time. There, we see a nuclear catalysis phenomenon of hydrogen transmutation into helium.
The cycle starts with a proton reaction with carbon, which results in the production of the nitrogen isotope 13N and the release of energy. The nitrogen nucleus then loses a positron and obtains a 13C carbon isotopic nucleus transformation. When the latter reacts with a proton, the isotope of nitrogen 14N is formed, accompanied by a new release of energy. Nitrogen then reacts with a proton to form the oxygen isotope 13O, which also releases energy. When an oxygen nucleus loses a positron, it becomes a 15N nitrogen nucleus, which reacts with a proton to produce the cycle's final product.
As a result, four 1H protons are transformed into a helium 4He nucleus; some of the nuclei produced in the intermediate reactions are unstable; the period of 13N is 10.1min, while that of 15 O is only 125 s. Thermal protons initiate this cycle in the depths of stars. Even at temperatures in the tens of millions of degrees, the relative quantity of thermal protons is insignificant; additionally, the resumption of successive cycles is slow. Given a specific hydrogen nucleus, it takes several million years for it to participate in the cycle and complete it by being incorporated into the helium nucleus; however, the number of protons within stars is so great that even this very slow pace of the cyclic processes is sufficient to ensure the Sun's activity for 30 billion years.
- It should be noted that an acceleration of this reaction has the potential to raise the temperature of the Sun. A rise in temperature causes the solar mass to expand, lowering the concentration of protons and thus slowing the carbon-nitrogen cycle. All forms of life on Earth are in some way dependent on solar energy; hence, the transformation of hydrogen into helium that occurs in cosmic space is intricately tied to our existence, to the past, present, and future of Life on our planet.
References:
Interstellar Atoms and Molecules
Star Formation- University of Rochester
Interstellar medium-Wikipedia
THE COSMIC CHEMICAL BOND-David Arnold Williams T W Hartquist
EARTH AND COSMOS-Robert S Kandel
Radiation as a Constraint for Life in the Universe-Ximena C.AbrevayaBrian C.Thomas
What elements make up the Sun?- Kristine Spekkens
Our Sun- NASA science
- Apfel, Necia, Nebulae: The Birth and Death of Stars, 1988, Lothrop, Lee and Shepard, ISBN 0-688-07229-1. Explains the life cycle of stars to upper elementary school students and above (Book).
The Chemical Composition of Stars and the Universe
Comets- NASA science
White dwarf
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.
Nice blog. It was interesting to read. I, as for the last time, have a couple of comments.
Note that the most abundant form of matter in the universe is not hydrogen, but dark matter. This is for some reason very often forgotten (maybe because it is off topic, somewhat ;) ).
The fusion reaction you described is not the only occurring in the Sun. There are indeed several ways to produce helium-4 (see the proton-proton chains I, II and III). The one dominating in our Sun is the second chain, that proceeds through reactions involving beryllium.
Thanks for these valuable notes Sir:
To be honest, I don't know enough about dark matter, therefore I'm reading your articles to understand more about it. However, most sources, particularly books, simply mention hydrogen as the most abundant substance in the universe. This is what made me think that hydrogen is the most prevalent substance in the universe, now I will correct my information and thank you very much
This is indeed the case. And this is true not only for books, but also for many scientists. This is however not completely true. It is the most abundant visible substance in the universe. The extra adjective makes a large difference, and renders the sentence correct :)
Thank you sir, greetings to you
Congratulations @benainouna! You have completed the following achievement on the Hive blockchain and have been rewarded with new badge(s):
Your next target is to reach 8000 upvotes.
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPCheck out the last post from @hivebuzz:
Support the HiveBuzz project. Vote for our proposal!
Dear @benainouna, we need your help!
The Hivebuzz proposal already got an important support from the community. However, it lost its funding few days ago and only needs a few more HP to get funded again.
May we ask you to support it so our team can continue its work this year?
You can do it on Peakd, ecency, Hive.blog or using HiveSigner.
https://peakd.com/me/proposals/199
Your support would be really helpful and you could make the difference! Thank you!